

Other combinations of cards will require different responses. If a reaction occurs, for example if magnesium and copper nitrate are shown, the first student to shout ‘reaction’ wins the cards. Then, at the same time, the two students place their next cards face up. In pairs, students shuffle the cards and deal out an equal number to each person. Students can consolidate their use of the reactivity series with a game of Displacement reaction snap. Ask students to write word equations for the reactions they observe, and justify them with reference to the reactivity series. They should be observing the production of new metals – copper forming on more reactive metals tends to be the most obvious observation, along with the change in colour of the solutions. Students may incorrectly identify this as a displacement reaction. This can occur as some of the solutions are slightly acidic and the reactive metals will evolve hydrogen. It may be worth demonstrating a couple of reactions using a visualiser beforehand, especially those that tend to bubble. Provide a couple of sieves over jugs for students to tip their reaction mixtures into at the end of the practical.ĭisplacement reactions produce new metals, such as when iron is immersed in copper sulfate solution (right) – but not when copper is put in iron sulfate solution (left) The reactions can be carried out on drop tiles, or on the integrated instruction sheets inside plastic wallets. If you can, get the solutions in dropper bottles as this will minimise the risk of cross-contamination. This will limit the volumes of solutions required, and simplify the clearing-up process. This is best carried out as a microscale reaction, using only a few drops of the solutions and small pieces of the metal. The key practical to carry out here is investigating the reaction of metals with metal compound solutions. This will also give them a preview of the link between metal reactivity and electricity, useful when discussing extraction by electrolysis. You could adapt the Electricity from chemicals practical, by setting up different cells around the laboratory for the students to collect data. Practical chemistryĪ quick way for students to confirm the validity of the reactivity series is by using basic electrochemistry.
Reactivity series mnemonic how to#
Knowing how to use the series would also be helpful. Having an idea of where groups of metals are on the series could be helpful, for example being aware that the more reactive groups are at the top and jewellery metals are at the bottom. However, this isn’t necessary, and could be just as off-putting as learning the periodic table may be. Some students will want to learn the series – developing mnemonics for this can be helpful. Provide students with a copy of the reactivity series, which they can refer to over time. The tales of Humphry Davy’s experiments at the Royal Institution provide fascinating insights into the development of scientific knowledge. These are placed above carbon in the reactivity series. The discovery and use of electricity was required to extract these reactive metals (group 1, group 2, aluminium).
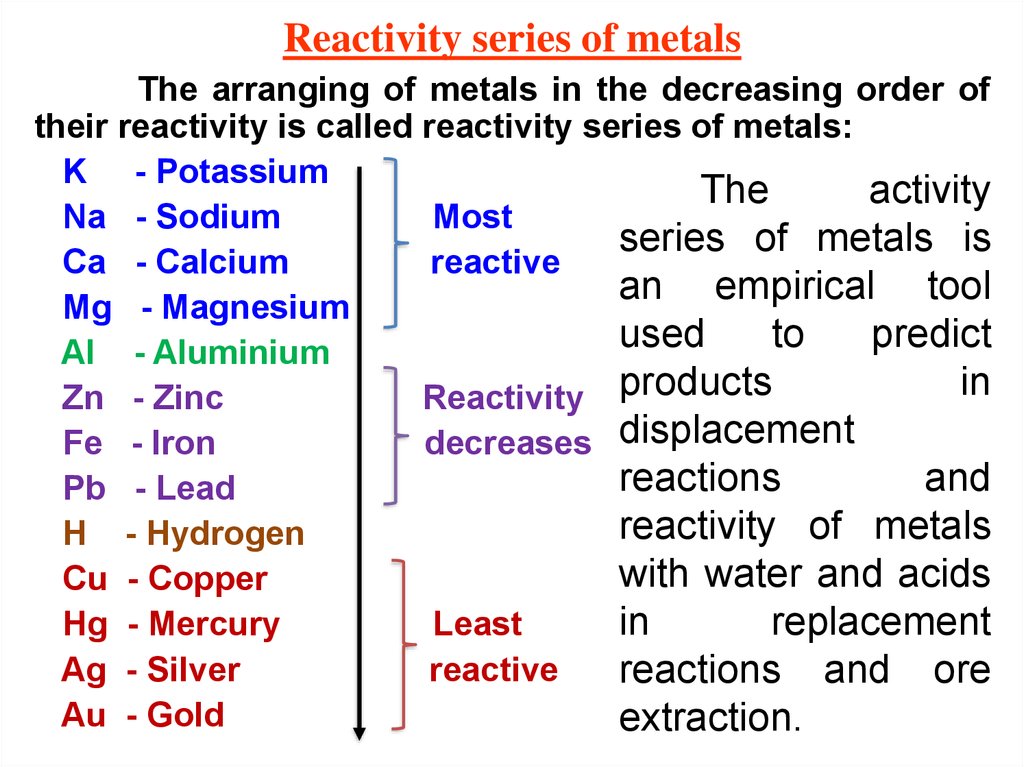
Reactivity series strips, and a Displacement reaction grid for formative assessment integrated instructions for a microscale practical investigating the reaction of metals with metal compound solutions and Displacement reaction snap from the Education in Chemistry website: rsc.li/2OfpmtQĪs previous generations of chemists discovered, many metals cannot be extracted with carbon.


 0 kommentar(er)
0 kommentar(er)
